—Vanessa Edwards, Michael Lyles, B1Daily
President Trump signed an executive order early in his term to create a Make America Healthy Again Commission aimed at addressing the nation’s chronic disease burden, and that effort explicitly mentioned accelerating research for genetic treatments for conditions like sickle cell disease. This establishes the administration’s public interest in tackling the disease within a chronic-illness framework.
What is Sickle Cell?
Sickle cell disease is a heritable blood disorder that disproportionately affects Black Americans — roughly **90% of U.S. patients — and leads to painful crises, organ damage, and shortened life expectancy.
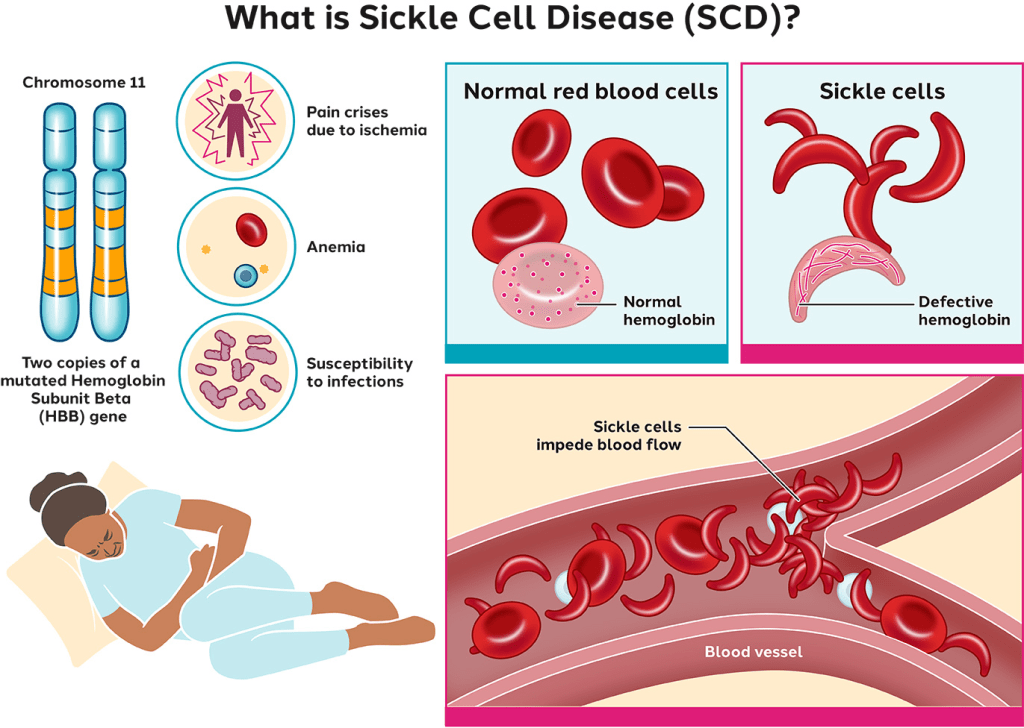
Historically, access to cutting-edge treatments like gene therapy has been limited by extremely high upfront costs, Medicare and Medicaid coverage barriers, and uneven clinical infrastructure across states. By negotiating outcome-based payment agreements, the Trump/Oz-led CMS model seeks to reduce upfront pricing concerns and encourage states to adopt therapies for eligible patients — potentially improving access for people on Medicaid who might otherwise be excluded due to cost.
What the CMS Cell and Gene Therapy Access Model Does
Under Trump-aligned leadership at CMS, including Dr. Oz, the agency expanded a model to increase access to innovative treatments for sickle cell disease:
Key Features
The Cell and Gene Therapy (CGT) Access Model partners with 33 states and territories to negotiate outcomes-based agreements with gene therapy manufacturers. This means states pay for treatments only if they work as promised.
It includes incentives for states to implement and track these outcomes, and potential federal support grants to help with rollout.
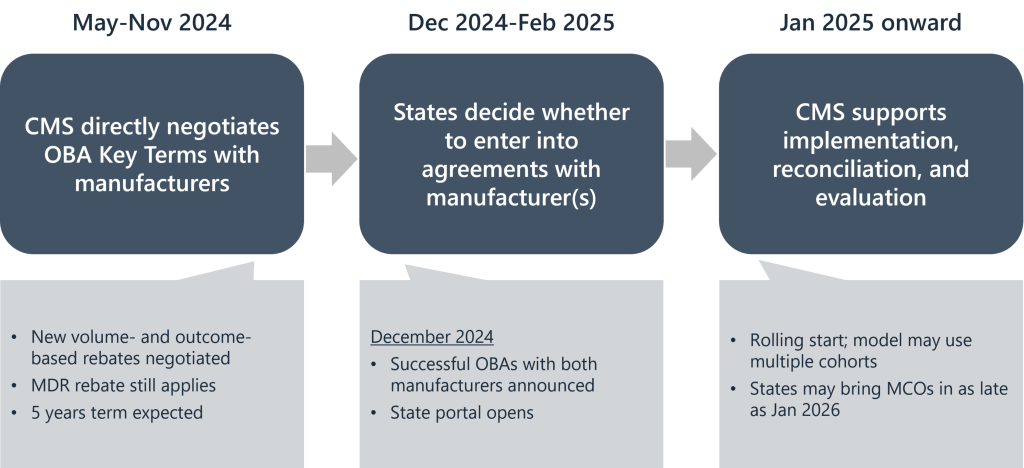
The goal is to lower barriers to high-cost but potentially transformative therapies for SCD that have recently been approved by the FDA.
This model is distinct from traditional fee-for-service Medicaid reimbursement: it ties payment to real world clinical results, which supporters argue can expand access while protecting taxpayers from paying million-dollar therapy prices without proven benefit.
Critics say that participation in the model is voluntary for states and manufacturers, meaning not all patients or therapies will be covered immediately. Additionally, negotiating payments is just part of the battle; many states and hospitals still lack infrastructure and trained specialists to deliver complex gene therapies. This bottleneck can constrain the impact of any federal pricing model. Independent reporting has noted cuts to NIH funding under the Trump administration that have affected sickle cell research, raising questions about long-term support for understanding and treating the disease beyond payment models.

The Trump administration, with Dr. Oz in a leadership role at CMS, has pushed forward a small-scale access model that could expand use of life-changing sickle cell therapies by tying payment to outcomes and reducing financial barriers for state Medicaid programs. This marks a noteworthy shift toward payment innovation for rare diseases. However, challenges remain in ensuring broad patient access, building clinical infrastructure, and maintaining robust research support — especially in the context of other federal budget decisions that have impacted disease research funding.
—Vanessa Edwards, Michael Lyles, B1Daily





Leave a comment